Mirena IUD Migration Lawsuit Joins NY MDL
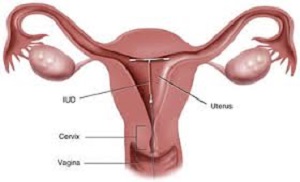 Another Mirena IUD migration lawsuit has joined the multidistrict litigation (MDL) in New York state, adding to the well over 1000 cases consolidated there over the allegedly defective form of birth control, as well as to the hundreds of cases that are part of a state-level multi-county litigation (MCL) in New Jersey.
Another Mirena IUD migration lawsuit has joined the multidistrict litigation (MDL) in New York state, adding to the well over 1000 cases consolidated there over the allegedly defective form of birth control, as well as to the hundreds of cases that are part of a state-level multi-county litigation (MCL) in New Jersey.
The plaintiff, Patricia Brewer, is a resident of Illinois who initially filed her complaint over the Mirena IUD in that state on September 17 of last year. Brewer v. Bayer Healthcare Pharmaceuticals Inc. et al (Case 7:14cv9896) was then transferred to the U.S. District Court of Southern New York (White Plains), where the Mirena IUD MDL is taking place under the Honorable Cathy Seibel, on December 16.
Preliminary (or “bellwether”) trials are slated to take place in 2016 in order to test the waters for subsequent cases and set a precedent for them.
Injuries, pregnancy detailed in latest Mirena lawsuit
The plaintiff received the IUD at Rush University Medical Center in Chicago on May 13, 2004. The initial procedure was relatively problem-free and the plaintiff followed the doctor’s advice regarding self-monitoring. She was also seen, appropriately, for follow up visits by the doctor.
Despite using the device as indicated, the IUD failed as a form of birth control and the plaintiff gave birth to a child via c-section on February 21, 2007. During the birth, the plaintiff’s bladder was lacerated. In subsequent follow up examinations, it was determined that the IUD had migrated outside the uterus. The plaintiff underwent surgery on Nov. 7, 2007 to remove the device.
The plaintiff did not, initially, connect her conception and subsequent medical problems with a defect in the device. However, she was made aware of problems with the Mirena IUD through a television commercial that she saw which detailed the problematic nature of the IUD. She filed a lawsuit within two years of becoming aware of the harm that it allegedly had done.
In addition to compensation for her injuries, medical expenses, and other costs, Brewer is suing for “further personal injuries [which] include, but are not limited to, pain and suffering, permanent bodily impairment, mental anguish and diminished enjoyment of life.”
Defendant accused of misrepresenting safety of product
Like other Mirena lawsuit complaints registered by plaintiffs in the MDL, Brewer’s complaint points out that Bayer has been accused of misrepresenting both the effectiveness and the safety of their product.
For instance, in their “Busy Moms” advertising campaign, the defendant claimed that users of the IUD would enjoy greater marital intimacy and improved appearance, none of which has been substantiated through reputable studies. On the other hand, the warning label includes no reference to the possibility of spontaneous Mirena migration and suggests that any kind of migration would be “uncommon.”
In contrast, the defendant has received numerous accounts of migration and organ perforation associated with Mirena since its approval as a birth control method by the FDA in 2000, but failed to appropriately report them.
- Newsweek, The Courtroom Controversy Behind Popular Contraceptive Mirena http://www.newsweek.com/2014/05/02/courtroom-controversy-behind-popular-contraceptive-mirena-248443.html
- Drugs.com, Mirena http://www.drugs.com/pro/mirena.html
- FDA, Mirena (levonorgestrel-releasing intrauterine system) July 2008 http://www.fda.gov/Safety/MedWatch/SafetyInformation/Safety-RelatedDrugLabelingChanges/ucm121936.htm


 Resources
Resources
 Resources
Resources
