New Case Study Highlights Risks of Mirena Side Effects
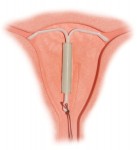 A recent case study involving Mirena side effects highlights the risks associated with this method of contraception. A 26-year old woman had to have the intrauterine device (IUD) removed from her abdomen after she discovered she was 12 weeks pregnant. The device had migrated outside the uterus, where it remained undetected for a number of years.
A recent case study involving Mirena side effects highlights the risks associated with this method of contraception. A 26-year old woman had to have the intrauterine device (IUD) removed from her abdomen after she discovered she was 12 weeks pregnant. The device had migrated outside the uterus, where it remained undetected for a number of years.
Misdiagnosis made due to migration of the device
The woman had the device inserted into her uterus nearly four years prior to the pregnancy. After the IUD was implanted, the woman realized the guide strings that indicate the device is in the proper place went missing. Her physician performed an ultrasound, but could was unable to find the Mirena IUD inside her uterus. It was then determined that the device must have fallen out of the woman’s body.
Unfortunately, a Mirena uterine perforation had occurred instead, and the device had migrated to another area of the woman’s abdomen. The device went undetected for a number of years, until abdominal pain brought the woman back to her doctor’s office. At this point, the woman had a viable uterine pregnancy, and the decision was made to remove the device surgically to prevent further Mirena side effects and pregnancy complications.
Risk of Mirena uterine perforation higher than indicated
Although the procedure was successful in this case study, even the physicians performing the procedure questioned their decision to remove the device during the woman’s pregnancy. Removal of the IUD carried its own risks, but doctors were also concerned that leaving the device in situ could result in infection or organ injury. Removal could prohibit risks to the fetus and mother from hormones released by the IUD during pregnancy and lower the risk of a septic pregnancy or miscarriage.
Despite the risks, doctors treating the patient had no way of knowing for sure if the IUD would actually result in any unwanted side effects during pregnancy. There were also risks involved with the surgery to remove the device, particularly in light of the patient’s current condition. Even after the procedure, doctors involved continue to question whether surgical removal was the most prudent decision at that time.
Mirena IUD injuries not uncommon
This is not the first complication involving a Mirena IUD. The device was approved by the FDA in 2000 as a means of contraception that could last for up to five years. However, reports of Mirena side effects, including migration of the device outside the uterus have been received by the FDA. In some cases, the migration occurred long after the device was inserted, despite product packaging that suggests most migrations occurred during the insertion process.
If the IUD migrates outside of the uterus, the woman can experience pain, infection and damage to surrounding organs. The device must be located using ultrasound technology and then surgically removed. Surgical procedures carry their own risks, including infection, pain and organ damage. Some women who have experienced these side effects have been unable to have biological children after the device was removed.
Women who become pregnant while the Mirena IUD is inserted face additional risks during pregnancy, including a septic pregnancy, ectopic pregnancy and miscarriage. Ectopic pregnancies occur when the fertilized egg begins development in an area other than the uterus, such as the Fallopian tube. A fetus cannot survive in the case of an ectopic pregnancy, and if the condition is not promptly diagnosed and treated, it can be dangerous for the mother as well. The rate of ectopic pregnancies in women with the Mirena IUD may be as high as 50 percent.
Today, injured women are bringing their claims against manufacturer Bayer to courtrooms across the country. These women are alleging Bayer downplayed the potential risk of Mirena uterine perforation while overstating product safety and benefits. Women are seeking compensation from Bayer for medical bills, pain and suffering and lost wages.

 Resources
Resources
 Resources
Resources
