Rates of Copper vs. Mirena IUD Organ Perforation Compared
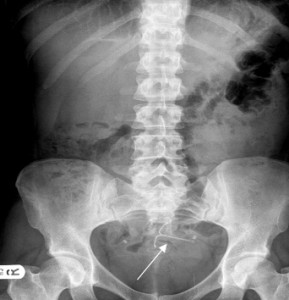 A recent study comparing rates of copper IUD (Cu-IUD) vs. Mirena IUD organ perforation found no significant difference between the two types of birth control devices in this regard, with the risk of perforation incidences relatively low for each (around 1 per 1,000 insertions).
A recent study comparing rates of copper IUD (Cu-IUD) vs. Mirena IUD organ perforation found no significant difference between the two types of birth control devices in this regard, with the risk of perforation incidences relatively low for each (around 1 per 1,000 insertions).
Over 61,000 women from six different countries were included in the study, which authors called the European Active Surveillance Study for Intrauterine Devices (EURAS-IUD). To date, it is the largest study of IUD efficacy, adverse events, and risk factors. The study was published on-line early this month in the journal Contraception.
Copper vs. hormonal IUD organ perforation
The study followed the women between 2006 and 2013. They filled out a baseline questionnaire at the beginning of the study and, in addition, both the women and their health-care professionals completed a follow-up questionnaire after 12 months. The objective of the study was to track the incidence of adverse events associated with IUD use, including perforation, under normal conditions, and to compare two leading types of IUDs in this regard.
Some 70% of the women in the study used the Mirena IUD, while 30% used a Cu-IUD. All in all, the study found that 81 of the women experienced uterine perforations: 61 among the Mirena IUD users and 20 among the copper IUD users. The resulting adjusted risk ratio between the two types of IUDs was not statistically significant.
Study compares IUD effectiveness, rates of ectopic pregnancy
The EURAS-IUD study also tracked other types of adverse events, including ectopic pregnancies, as well as risk factors, and the overall effectiveness of each type of IUD as a birth control method generally and comparatively.
The study found that while both types of IUD were extremely effective at preventing pregnancy, users of the copper IUD were eight times more likely to experience an unplanned pregnancy (5.2 vs. 0.6 pregnancies each for every 1000 women).
For both devices, rates of ectopic pregnancies were low among those women who did get pregnant. There were 118 pregnancies, with 21 of them ectopic (7 for the women using the Mirena IUD and 14 for the women using the copper IUD).
Of the 81 incidences of perforation, 63 were associated with risk factors or suspected risk factors. These included breastfeeding, and an occurrence in fewer than 36 weeks since the time of the last delivery. In each of the 81 cases, the perforation did not result in a serious illness or in the puncture of other organs or pelvic structures.
Mirena IUD organ perforation rates
The latest study stands in contrast to previous findings from scientific studies and FDA reports that suggest Mirena IUD organ perforation may be a more serious problem.
For instance, a University of California radiology department study suggested that IUD migration and perforation may be “frequently occurring complications,” though no specific statistics were given. Between 2000 and 2013, the FDA has received 70,000 adverse event reports regarding the Mirena IUD, though only some of these incidents include perforation. FDA records also include references to 4,775 women who have experienced device dislocation since 2008, with 1n322 of these women also having experienced uterine perforation.
Hundreds of women who have taken legal action against the manufacturer of the Mirena IUD have reported incidences of uterine perforation and other complications, with many claiming to have suffered intense pain and discomfort or even more dramatic complications, such as a punctured liver.
- Contraception, Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices http://www.contraceptionjournal.org/article/S0010-7824(15)00008-6/fulltext
- FirstWordPharma, Study shows Mirena (LNG-IUS 52mg) significantly more effective than copper IUD in preventing pregnancy http://www.firstwordpharma.com/node/1261828#axzz3R5g97cK5


 Resources
Resources
 Resources
Resources
