Discovery Schedule Proposed for Bard IVC Filter MDL
A discovery schedule has been proposed for the Bard IVC filter lawsuits currently pending under multidistrict litigation in Arizona.
The schedule pertains to some of the initial cases filed in the MDL and should allow for the first federal trials to get underway during the early months of 2017.
Approximately 100 product liability lawsuits have been filed under the MDL. Plaintiffs site similar allegations that the design of the IVC filters is seriously flawed and has an excessively high risk of failure. Many claim they were severely injured when the IVC filters migrated out of position, punctured the vein or fractured — which in some cases sent small metal particles to the lungs or heart.
Bard IVC filter MDL
The Bard IVC filter MDL was established in August 2015 in the District of Arizona. U.S. District Judge David G. Campbell is overseeing the litigation. The MDL was created as a way to reduce duplicate discovery, conserve resources for both sides and to avoid conflicting pretrial rulings.
Currently, only 100 cases have been joined together under the MDL, but this number is expected to grow exponentially. In total, several thousand complaints may be centralized under the MDL, but many of the early cases had already reached the advanced stages of litigation before August 2015, when the MDL was established.
Two separate discovery tracks proposed
In late January, both parties presented a joint report containing a proposal of two separate discovery tracks to Judge Campbell. One track would include cases already in the advanced stages of litigation and the other would contain newer cases and any additional ones filed in the future.
Under the terms of the first track, all discovery would need to be completed by July 1 of this year. The parties involved would then trade supplemental reports from experts and the deadline to complete additional expert discovery would be Nov. 4. Consequently, these cases would be ready for trial by the beginning of 2017.
The second group calls for a parallel discovery track with a slightly extended timeline. Under the terms of this track, general discovery would close on Oct. 28 of this year and common-issue expert discovery would commence on May 19, 2017.
The parties have indicated that preliminary discussions for the bellwether selection process have already taken place. This small group of representative cases is designed to help determine how juries may respond to evidence and testimony that may be repeated in a number of cases. The outcome of the bellwether trials will not be binding on other cases, but they could influence future negotiations between the plaintiffs and Bard.
The joint reported noted that the parties expect to present their next submission on March 1, which will detail their plan for the bellwether process and other related information.
FDA warns of IVC filter risk
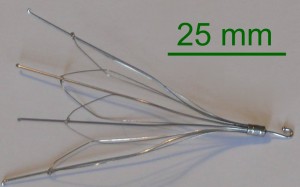
A vena cava filter is an expandable metal device that is implanted in the inferior vena cava to prevent blood clots from reaching the lungs — known as a pulmonary embolism. IVC filters are designed for permanent placement in the body, but some have the option for removal.
On May 6, 2014, the U.S. Food and Drug Administration issued a safety communication acknowledging reports of adverse events and issues associated with IVC filters. Complications included filter fracture, device migration, perforation of the IVC, problems removing the device and embolization.
The agency advised that some of these concerns could be related the length of time the filter has been in the body. Patients with retrievable filters were advised to have them removed after the risk of pulmonary embolism decreases, to lower the possibility of developing IVC filter complications.
- U.S. District Court for the District of Arizona, Bard IVC Filters Products Liability Litigation http://www.azd.uscourts.gov/case-info/bard
- Bard Peripheral Vascular, Bard Inferior Vena Cava Filters http://www.bardpv.com/filterfacts/filterfacts.html
- U.S. Food and Drug Administration, Removing Retrievable Inferior Vena Cava Filters: FDA Safety Communication http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm396377.htm


 Resources
Resources
 Resources
Resources
