Health Canada Issues IVC Filter Warning
 Health Canada has issued a warning regarding inferior vena cava (IVC) filters. The agency has alerted healthcare providers to the fact that these filters can lead to serious complications, including perforation of the vein by the filter, fracture of the filter and patient death. The IVC filters have also become a source of concern in the United States and patients that have been injured by the filters have begun filing lawsuits seeking damages for their injuries.
Health Canada has issued a warning regarding inferior vena cava (IVC) filters. The agency has alerted healthcare providers to the fact that these filters can lead to serious complications, including perforation of the vein by the filter, fracture of the filter and patient death. The IVC filters have also become a source of concern in the United States and patients that have been injured by the filters have begun filing lawsuits seeking damages for their injuries.
Dozens of reports of adverse events
The new warning from Health Canada was issued on July 25, 2016, after the agency received 121 reports of serious complications related to these devices. In addition to the complications listed above, some patients have had the filters perforate their heart. In the event of a filter fracture, pieces of the filter can travel to various areas of the body, in some cases getting lodged in vital organs like the heart or liver. These types of complications can be life-threatening for patients.
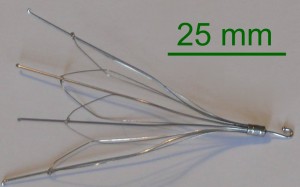
IVC filters are thin metal devices designed to fit inside the inferior vena cava, the largest vein of the body. When placed inside, the filters prevent blood clots from moving up the lower leg and traveling to the lung, where they can cause a deadly event known as a pulmonary embolism. They are recommended for patients with deep vein thrombosis (DVT) that are unable to manage their condition effectively through medication or other therapies.
Unfortunately, the very devices that were created to increase safety have actually posed a new danger for some patients. One of the IVC filter risks, as noted by both Health Canada and the FDA, is that the filters are often left in the vein longer than intended. While it is recommended to remove the filter as soon as the threat of pulmonary embolism is passed, some of these devices are being left in the vein much longer. This can increase a patient’s risk for serious complications and make the filter more difficult to remove completely and safely.
IVC filter lawsuits in U.S., Canada
Like the United States, patients in Canada have also been filing IVC filter lawsuits against the manufacturers of these devices. Plaintiffs are alleging their filters have broken and become trapped in their bodies. Others have experienced migration of the device to other areas of the body. Plaintiffs are also claiming the manufacturers did not provide sufficient warning about the risks associated with the devices or the need to remove the devices in a timely fashion.
The Health Canada warning list includes 12 IVC filters made by six different manufacturers. Although the agency does acknowledge these filters can be a life-saving treatment for some patients with DVT and a heightened risk of pulmonary embolism, it stresses the importance of removing the device within 30 days of implantation.
As a response to the warning, doctors at Toronto General Hospital have made efforts to follow up with patients that have received the IVC filters and determine whether some of those filters need to be removed. Studies at the hospital have shown that only about 41 percent of the filters implanted there have been successfully removed over a 12-year period. The hospital is the first in Canada to take such steps.
- Government of Canada, Inferior Vena Cava (IVC) Filters – Risk of Serious Complications, http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2016/59518a-eng.php
- CTV News, Health Canada Issues Warning over Blood Clot Filters, http://www.ctvnews.ca/health/health-headlines/health-canada-issues-warning-over-blood-clot-filters-1.3020564
- Yahoo Finance, Health Canada Issues Safety Warning on IVC Filters, http://finance.yahoo.com/news/health-canada-issues-safety-warning-203300066.html
- Canadian Family Physician, Using Inferior Vena Cava Filters to Prevent Pulmonary Embolism, http://www.cfp.ca/content/54/1/49.full
- FDA, Removing Retrievable Inferior Vena Cava Filters: FDA Safety Communication, http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm396377.htm


 Resources
Resources
 Resources
Resources
